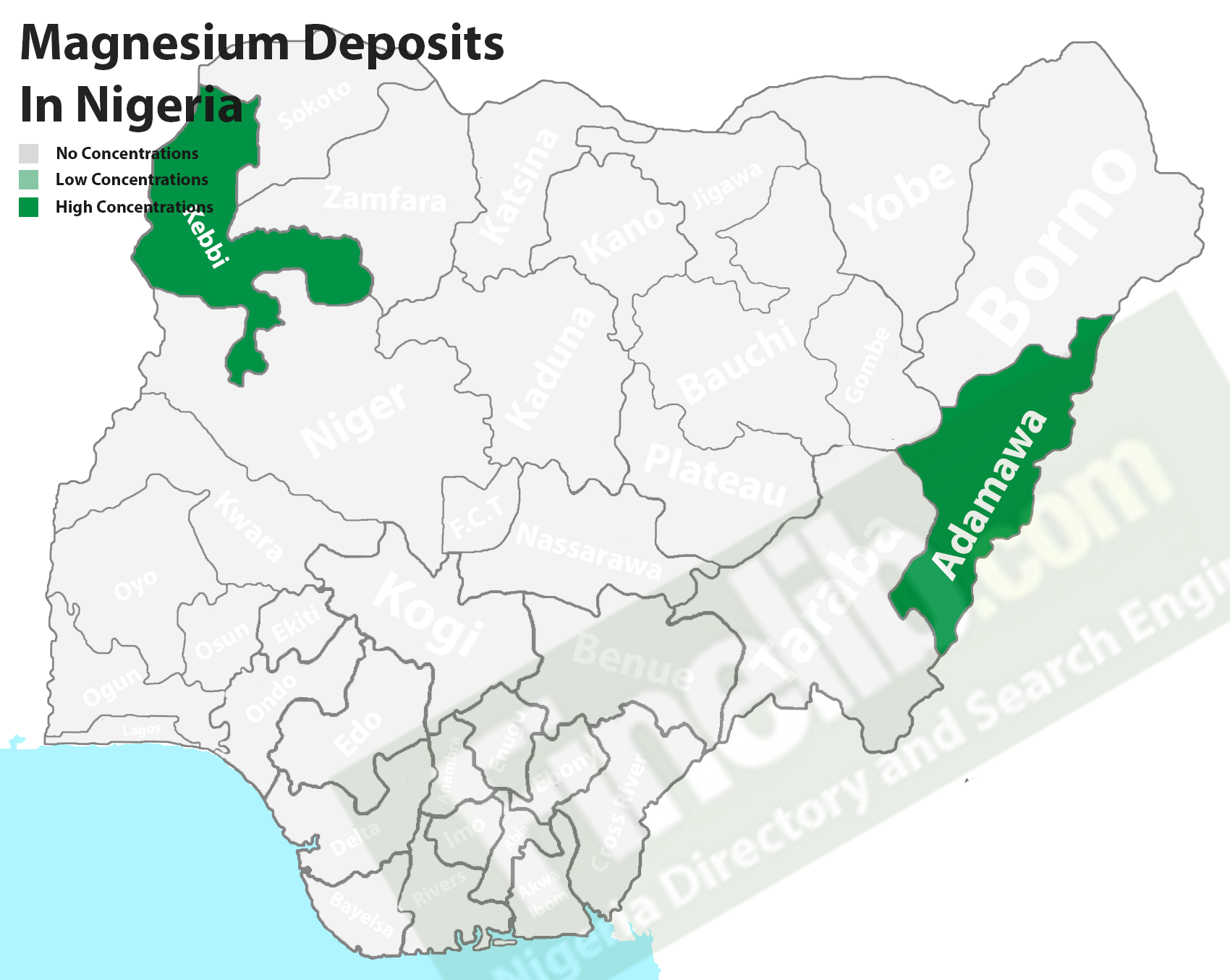
Nigerian States With Magnesium (Mg) Deposits

Magnesium can be found in Nigeria but in fewer locations unlike some African countries with vast deposits, it is one of the mineral resources that occur in nature in combination with other minerals. It is deposited in Adamawa and Kebbi states.
Magnesium has a symbol of Mg with an atomic number 12; It is in group 2 (alkaline earth metals) of the periodic table, and of all the alkaline earth metals it has the lowest melting point of 650 °C and also the lowest boiling point of 1,091 °C.
It has a shiny grey solid (silvery-white) metal that ignites easily in air and burns with a bright light; It is the 8th most abundant element in the universe, the 4th most common element on Earth after iron, oxygen, and silicon, the 3rd most abundant element dissolved in seawater, after sodium and chlorine, and the 11th most abundant element by mass in the human body and makes up about 13% of the planet's mass.
It only occurs in nature in combination with other minerals such as magnesite and dolomite.
Magnesium can be produced using the thermal reduction process and electrolytic process
a. Electrolytic process
The electrolytic process is mostly used in the United States to produced magnesium from magnesium chloride, and this occurs when seawater or magnesite is subjected to treatment together with calcium oxide, the reason for the calcium oxide is to help the resulted magnesium hydroxide to precipitate out. When the precipitate is collected, it is further treated with hydrochloric acid and heated to electrolyzed the compound converting it into magnesium chloride (magnesium and chlorine gas).
b. Thermal Reduction Process
Consist of two processes, namely:
I. Silicothermic process
In using the silicothermic process, magnesite or calcined dolomite is heated to the point of vaporization after it has been mixed with ferrosilicon alloy, the vapor (magnesium vapour) is collected and then allowed for some time to cool, the rate of the cooling can be faster when it is put in a cooling vessel making the vapour to condense to magnesium, this process is mostly used in China.
II. The third process is carried out by dissolving magnesite in hydrochloric acid to make magnesium chloride which is purified, dehydrated, and electrolyzed in an Alcan cell. The magnesium when in a molten state is removed and the chlorine gas (the leftover) is recycled and combined with hydrogen to make more hydrochloric acid. The process is mainly used in Australia.
Uses of magnesium
- Magnesium is the metallic ion at the centre of chlorophyll and is a common component in fertilizers.
- Magnesium is alloyed with aluminium and small amounts of copper, nickel, and tin to produce magnalium.
- Duralumin is an alloy of aluminium-alloy, copper, manganese, and magnesium.
- It is useful in an airplane and car construction when used as an alloying agent.
- It is also used in pyrotechnics, like incendiary bombs, fireworks, sparklers, flares, and as a fuse for thermite because it is easily ignited and burns with a bright light.
- It is used in photographic flashbulbs and is added to some rocket and missile fuels.
- It is used in the preparation of malleable cast iron.
- It is used as a mordant for dyes.
- Magnesium hydroxide is used to produce plastics which gives it a fire retardant nature.
- It is also used in the making of heat-resistant bricks for furnaces and fireplaces.
- Because of its nutritional value to plants and animals, it is added to fertilizers and cattle feed respectively.
- Magnesium hydroxide is used in medicine including chloride, sulphate, and citrate.




